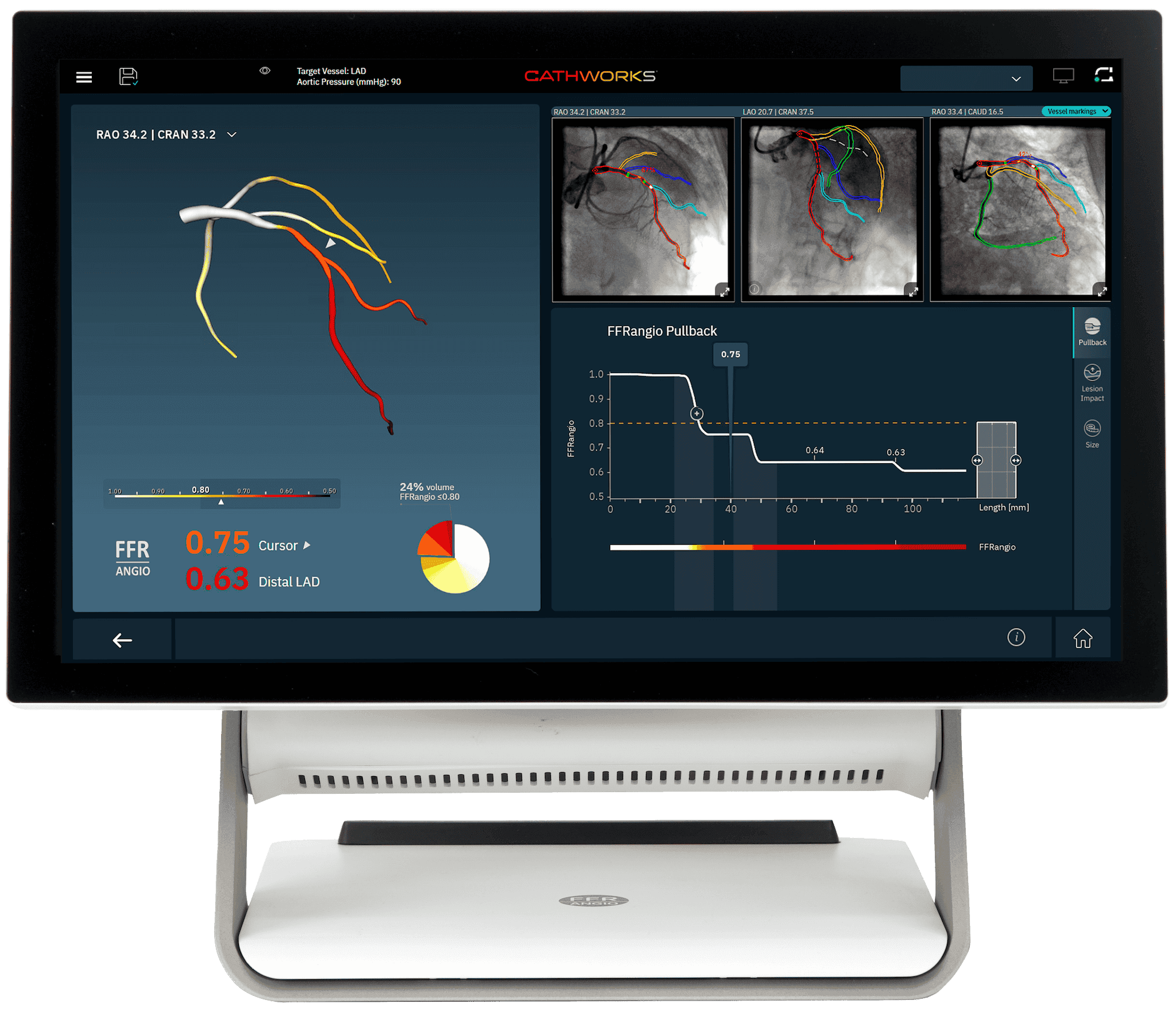
The CathWorks System provides advanced procedural modeling and comprehensive data integration—empowering clinicians with deeper insights for precision-driven decision-making. By leveraging a suite of innovative tools, CathWorks enhances workflow efficiency, optimizes procedural planning, and ultimately helps us deliver better outcomes for our patients.
Testimonials
Operators would prefer not to have to pull out a wire, deliver adenosine, or all the other things that come along with invasive measures, especially if they can get similar diagnostic accuracy and outcomes with FFRangio.
Thanks to FFRangio, interventional and referring physicians get a better understanding of the patient’s hemodynamics and physiology, rather than a quick assessment of the lesion and stent. This is much more detailed, and everyone wants more details.
FFRangio has accelerated the culture of collaboration in our cath lab and is one of the best products I’ve had in my professional career.
Optimize Clinical Decision Making with the CathWorks FFRangio® System

Hear from experts on how the CathWorks FFRangio® System can help optimize clinical decision making.
- Real-time FFRangio values at every point on the coronary tree
- Simulated pullback curve differentiates functional patterns of CAD to target the most important segments
- Lesion Impact enables excluding a lesion to assess residual ischemia*†
- Integrated sizing tool for non-invasive lesion measurements
*
Lesion Impact feature is only available in selected geographies.
†
Lesion Impact feature is not available for Diagonal and Ramus cases.
1.
Witberg G, De Bruyne B, Fearon WF, et al. Diagnostic performance of angiogram-derived fractional flow reserve: A pooled analysis of 5 prospective cohort studies. J Am Coll Cardiol Intv.2020;13(4):488-97